INTRODUCTION AND STUDY AIM
The roe deer Capreolus capreolus (Linnaeus 1758) is an eurytopic species of a great biological plasticity, able to live in various habitats. It inhabits large compact forest complexes, as well as woodless agrocoenoses, but most often it may be found in areas consisting of a mosaic of forests and cultivated fields. This smallest European representative of Cervidae is in Poland the most abundant, and at the same time the most common representative of wild ungulates. According to wildlife statistics [2] the roe deer population at the beginning of hunting season 2004/2005 numbered almost 670 thousand individuals, and according to Pielowski [8] its estimated actual numbers may reach as many as about a million animals.
Pelts of Cervidae are not of great importance in furriery, and they are only used for decorative purposes [3]. This is due to the fact that their hairs are weak and fragile, and their skins are not too durable.
In the case of roe deer only characteristics of the hair coat used by hunters to determine the age, sex, and health state of individuals are quite well known, i.e. coat colors and time of a molt [6,9].
A short description of roe deer hair was given by Sokolov [10], who however measured hairs collected from the nape and chest only. Such a description seem to be little precise, because, as it was shown by Bubenik [1] using the white-tailed deer (Odocoileus virginianus) as an example, hairs of Cervidae are characterized by a great variation depending on the sample collecting place on the animal’s body.
For this reason the aim of this study was to describe in detail selected features of roe deer hairs of the summer as well as of the winter coat.
METHODS
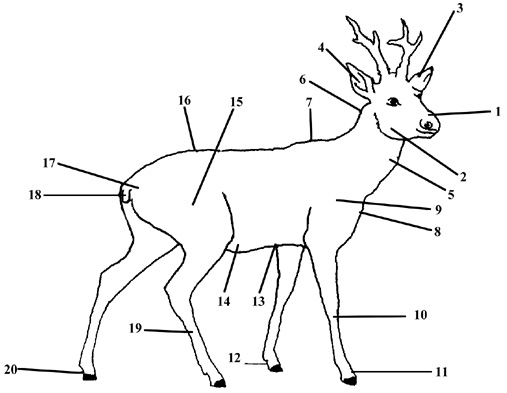
The field work was carried out in the area administered by the Forest Experimental Station in Krynica (49°20’ – 49°27’ N, 20°54’ – 21°07’ E) situated in the Beskid Sadecki range of the Carpathians. Hairs were collected from the summer coat (from 10 individuals) and from the winter coat (from 10 individuals) of adults of full color and without disease symptoms. Samples were taken from 20 different places on the body of each animal (Fig. 1). The hair was plucked out in such a way as to obtain 20 – 30 undamaged hairs.
Fig. 1. Topography of places on the roe deer body where hair samples were collected: region above nostrils (1), cheeks (2), external side of ear (3), internal side of ear (4), neck (5), nape (6), hip (7), chest (8), shoulder blade (9), metacarpus (10), base of digits (11), fetlock of forelimb (120, sternum (13), abdomen (14), thigh (15), back (16), rump (17), tail (18), metatarsus (19), fetlock of hind limb (20) |
 |
Measurements were taken from 5 hairs of each sample. Each time the following measurements were taken: hair length, thickness in the middle part of the hair shaft, length of the bulb, thickness of a hair at the contraction behind the bulb. The imprints of cuticular scales were made by submerging hairs in a polymer adhesive on a microscopic slide. Cuticular scales were counted on a constant length of a hair –70 µm. Attempts to obtain hair cross-sections by their cutting between two pieces of the black elder pith [4] were not successful because of susceptibility of the roe deer hairs to splitting and crushing. To avoid this problem several hairs were placed in troughs made of a corrugated paperboard and covered with a thin layer of cyanoacrylate adhesive. After drying it was easy to cut the hair tufts with a razor blade thus obtaining thin cross-sections.
The length of hairs was measured with a millimeter scale, while the remaining measurements, and other observations including photographs, were made under a light microscope at magnification from 90 to 400 x.
All statistical calculations were carried out using the program Statistica 6.0 PL [11]. significance of differences was estimated using the Duncan test, at the significance level p < 0.05 [31].
RESULTS AND DISCUSSION
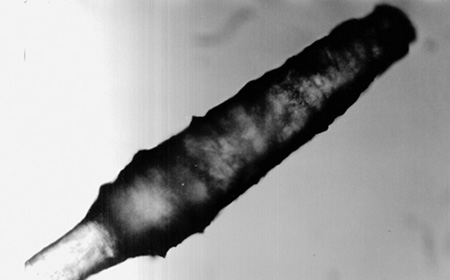
The microscopic observations permitted to conclude that the roe deer hairs have a granular medulla, which is a characteristic feature of the family Cervidae [4], and scales of the cuticle are arranged in a mosaic pattern. The thickness of the cortex is as a rule the same along the entire length of the hair shaft, and only the thickness of the medulla varies. The bulbs of hairs from the region above nostrils and from limbs are cylindrical and have rounded apexes (Fig. 2), while in the remaining hairs they are conical in shape and have sharper apexes (Fig. 3). The contraction behind the bulb is the thinnest place of the hair. Above this contraction the thickness of the hair suddenly increases reaching its maximum, and then it gradually decreases towards the apex. The hair pigment in the case of hairs with thin cuticle is present in the medulla, and in the case of hairs with a thick cuticle it is also present in the cortex giving it a dark color.
Fig. 2. The bulb of the hair from the metacarpus, summer coat, x160 |
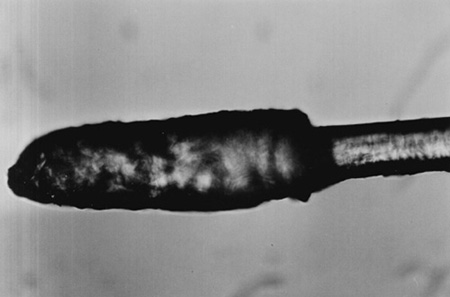 |
Fig. 3. The bulb of the hair from the nape, winter coat, x160 |
 |
Hairs of the winter season (Fig. 4) have cuticular scales by 40 – 50% larger than those of the summer season (Fig. 5). Hairs from limbs, the region above nostrils, the external side of the ear, and in the case of the summer coat also from cheeks, are different than the other hairs. Their cuticular scales are smaller, which may be interpreted by the fact that hairs with small cuticular scales are more resistant to abrasion and action of water. These hairs are also characterized by small seasonal differences in scale size. Scales in the winter coat are by 10 – 15% larger than those in the summer coat. No differences in scale pattern were observed along the hair shaft.
Fig. 4. Scales of the cuticle of hairs from the metacarpus, winter coat, x250 |
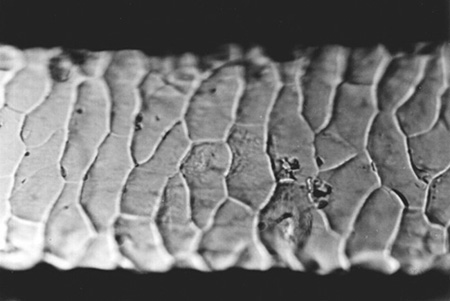 |
Fig. 5. Scales of the cuticle of hairs from the metacarpus, summer coat, x250 |
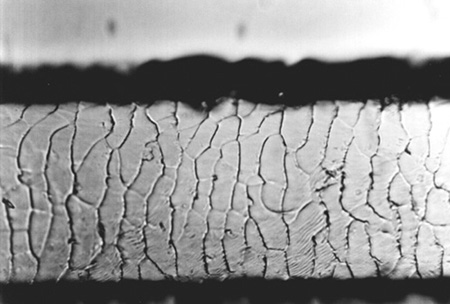 |
Detailed measurements of hair length and thickness are presented in Table 1. Mean values of hair length and thickness show seasonal variation which have been statistically proved. This variation is especially evident in vicinity of the trunk and neck (Fig. 1 – points 5 – 9, 13 – 17) where winter hairs are on the average by 7.06 mm longer and 92.3 µm thicker. This fact can be explained by a thermoregulatory function of hair and adaptation of animals, through molting, to life in the climate prevailing in Poland. Hairs of the internal side of the ear (point 4) are characterized by an untypical structure since all their measurements are greater in the summer season. Most likely this phenomenon can be explained by the function of these hairs, i.e. protection of the ear against dust and dirt which are more abundant in the summer. Another adaptation feature of these hairs, important for their protective function, is a relatively thick cortical layer. Such a structure makes hairs more elastic, and therefore less susceptible to felting, which would make them devoid of a protective ability.
| Table 1. Discriptive statistics of length and width of hairs (in the middle part of the hair shaft) in summer (S) and winter (W) coat, and results of the Manna – Whitneya U test on significance of differences between distributive series (α=0.05) |
|
Number of the place of measurement |
Length of hairs [mm] |
Thickness of hairs [µm] |
||||||||
|
S |
W |
Ho |
S |
W |
Ho |
|||||
|
|
SD |
|
SD |
|
SD |
|
SD |
|||
|
1 |
7.6 |
1.1 |
6.2 |
1.1 |
- |
64 |
9 |
94 |
14 |
- |
|
2 |
11.0 |
1.2 |
25.8 |
2.6 |
- |
84 |
16 |
133 |
14 |
- |
|
3 |
12.9 |
1.2 |
19.2 |
2.2 |
- |
74 |
10 |
72 |
18 |
+ |
|
4 |
32.5 |
3.3 |
19.3 |
2.1 |
- |
62 |
7 |
46 |
6 |
- |
|
5 |
21.0 |
2.0 |
31.8 |
3.1 |
- |
77 |
14 |
172 |
15 |
- |
|
6 |
27.2 |
2.8 |
37.6 |
3.6 |
- |
72 |
7 |
176 |
12 |
- |
|
7 |
28.2 |
2.8 |
35.8 |
1.9 |
- |
75 |
7 |
168 |
14 |
- |
|
8 |
28.3 |
2.7 |
28.4 |
3.0 |
+ |
89 |
11 |
158 |
11 |
- |
|
9 |
27.6 |
2.2 |
34.1 |
3.5 |
- |
95 |
6 |
169 |
15 |
- |
|
10 |
11.7 |
1.0 |
13.4 |
1.3 |
- |
76 |
11 |
88 |
10 |
- |
|
11 |
9.9 |
2.0 |
12.0 |
1.3 |
- |
61 |
7 |
84 |
14 |
- |
|
12 |
11.6 |
0.9 |
13.3 |
1.6 |
- |
58 |
6 |
61 |
7 |
+ |
|
13 |
26.6 |
1.6 |
36.0 |
4.2 |
- |
102 |
11 |
221 |
15 |
- |
|
14 |
26.6 |
1.6 |
36.6 |
2.6 |
- |
102 |
11 |
153 |
21 |
- |
|
15 |
32.6 |
2.0 |
35.9 |
3.3 |
- |
103 |
8 |
215 |
22 |
- |
|
16 |
33.4 |
2.3 |
39.0 |
3.9 |
- |
106 |
13 |
220 |
16 |
- |
|
17 |
43.8 |
2.2 |
52.0 |
5.1 |
- |
82 |
9 |
236 |
18 |
- |
|
18 |
45.2 |
2.2 |
33.8 |
2.8 |
- |
86 |
9 |
197 |
21 |
- |
|
19 |
10.1 |
1.1 |
13.3 |
1.8 |
- |
67 |
10 |
105 |
16 |
- |
|
20 |
12.7 |
1.3 |
14.2 |
1.4 |
- |
68 |
9 |
69 |
9 |
+ |
| Ho – zero hypothesis about equality of distributive series (“+” accept, “-” reject) |
The longest hairs, in the summer as well as in the winter coat, are present on a light spot on the rump (point 17). These hairs are bristled up in the case of danger, thus the spot on the rump becomes doubled in size. This is the alarm signal for other individuals in the herd, and also the sign for young animals following their mother [8].
The remaining measurements such as the thickness of the bulb (45 – 75 µm in the summer coat and 45 – 97 µm in the winter coat), length of the bulb (203 – 417 µm in the summer coat and 149 – 449 µm in the winter coat), and the hair thickness at the contraction behind the bulb (25 – 48 µm and 28 – 76 µm respectively) did not significantly differentiate the summer coat from the winter coat. While the Friedman’s test showed that hair parameters mentioned above are significantly diversified depending on the place of animal’s body from which hair samples were collected. Such a relationship was found for the summer as well as for the winter coat.
Measurements of hair cross-sections permitted to calculate the percentage of the medulla in the total area of a cross-section (Table 2). The aggregation of these results using the Euclidean distances permitted to distinguish the following groups of hairs:
group I – medulla makes 33.3% of the cross-section – hairs from the fetlock of the forelimb;
group II – medulla makes 61.5 – 80.0% of the cross-section – hairs from the region above nostrils, hind limb, and remaining hairs from the forelimb;
group III – medulla makes 87.0 – 92.5% of the cross-section – hairs from the ears and abdomen;
group IV – medulla makes 93.5 – 95.8% of the cross-section – remaining hairs (neck and trunk).
| Table 2. Mean percentage of the hair medulla in the total hair thickness of summer (S) and winter (W) coat |
|
Number of the place of measurement |
S [%] |
W [%] |
|
1 |
67.7 |
61.5 |
|
2 |
93.9 |
94.7 |
|
3 |
87.0 |
88.9 |
|
4 |
87.2 |
90.0 |
|
5 |
94.6 |
94.7 |
|
6 |
94.7 |
95.2 |
|
7 |
94.9 |
95.1 |
|
8 |
95.6 |
95.3 |
|
9 |
95.5 |
95.6 |
|
10 |
63.0 |
70.6 |
|
11 |
61.5 |
64.3 |
|
12 |
33.3 |
33.3 |
|
13 |
93.5 |
94.9 |
|
14 |
89.2 |
92.5 |
|
15 |
95.7 |
95.3 |
|
16 |
95.4 |
95.8 |
|
17 |
95.5 |
96.0 |
|
18 |
95.1 |
95.7 |
|
19 |
80.0 |
77.8 |
|
20 |
80.4 |
82.8 |
This classification perfectly shows the change of the basic function of hairs in transition from the hooves right up to the animal’s head. The general rule is as follows: the lower place on the animal’s body the stronger hairs but weaker insulating properties. There is a distinct increase in insulating properties from the limbs through the abdomen to the trunk and neck.
Hairs covering the trunk and neck play in the first place a heat-insulating role. They are adapted to this role thank to their considerable length as well as a thin cortical layer and strongly developed medulla with a large number of small closed spaces (Fig. 4). Such a structure aids the heat-insulating role, but makes hairs less durable [7]. The contraction behind the bulb, where there is no medulla, protects them against a quick breaking. This contraction permits hairs to deflect freely, thus increasing their durability. [10]. In roe deer the contraction behind the bulb is a very characteristic feature, as it is in form of a thin leg of constant thickness. In natural wear of hairs only their apexes are broken off, and this is demonstrated by the winter coat becoming more and more grey with passage of time [8].
Fig. 6. Cross-section of hairs from the nape, winter coat, x250, (1 – cortex, 2 – medulla) |
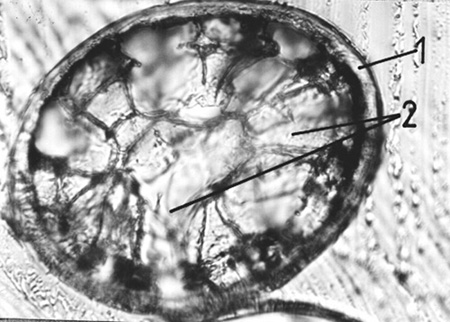 |
Fig. 7. Cross-section of hairs from the metacarpus, winter coat, x400, at the bottom the cross-section of the upper part of the hair is visible, (1 – cortex, 2 – medulla) |
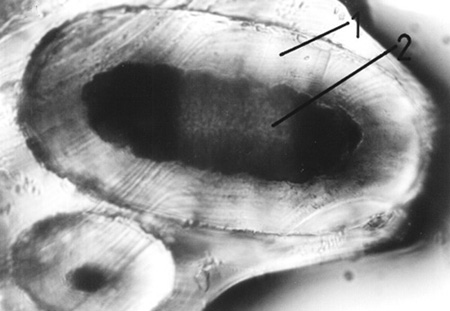 |
Hairs from the region above nostrils and from limbs have a characteristic structure, i.e. their cortex is strongly developed (Fig. 5), and their cross-section is ellipsoidal, which make them more durable and more resistant to mechanical damages. The remaining hairs are circular in their cross-sections.
CONCLUSIONS
On the basis of measurements carried out during this study considerable differences in hair structure between the winter and the summer coat were found. Hairs of the winter season are usually longer and thicker. In the case of length and thickness of the bulb and hair thickness in the contraction behind the bulb no such a simple relationship was found.
Within each season (summer, winter) significant differences in all analyzed elements (length and thickness of hairs and bulbs, hair thickness in the contraction behind the bulb) between hairs originating from different places on the animal’s body were found.
On the cross-sections of the hairs the medulla makes from 33.3% to 95.8% of their thickness. There was the dependence of this feature upon the place of hair origin. There is no medulla in the hair contraction behind the bulb nor in the hair apex.
Hair cross-section is ellipsoidal or circular, depending on the region of the body.
Scales of the cuticle having a mosaic arrangement are larger in the winter season.
REFERENCES
Bubenik G. 1996: Morphological investigations of the winter coat in white-tailed deer: Differences in skin, glands and hair structure of various body regions. Acta Theriol., 41, 1: 73 -82. Budna E., Grzybowska L., Żytecka-Karolak M. 2004: Lesnictwo 2004, Dział V Łowiectwo. Informacje i opracowania statystyczne. [Forestry 2004, Section V Wildlife Management. Information and statistical elaborations]. GUS Warszawa, 140-153 [in Polish]. Skóry surowe futrzarskie. [Raw furrier’s hides]. Akademia Ekonomiczna w Krakowie, Kraków, 1-240 [in Polish]. Dziurdzik B. 1978: Badania nad identyfikacja gatunków ssaków na podstawie budowy histologicznej włosów. [Studies on identification of mammal species on the basis of histological structure of hair]. Prz. Zool., 22, 2: 185-198 [in Polish]. Jarosz S. 1993: Hodowla zwierzat futerkowych. [Husbandry of fur-bearing animals]. PWN, Warszawa – Kraków, 1-274 [in Polish]. Lochman J. i in. 1987: Okreslanie wieku zwierzyny. [Determination of age of game animals]. PWRiL, Warszawa, 1-256 [in Polish]. Lutnicki W. 1988: Układ powłokowy zwierzat domowych. [Coat system of domestic animals]. PWN, Warszawa, 1-231 [in Polish]. Pielowski Z. 1999: Sarna. [Roe deer]. Oficyna Edytorska Wydawnictwo Swiat, Warszawa, 1-142 [in Polish]. Pucek Z. (red.) 1984: Klucz do oznaczania ssaków Polski. [The key for identification of mammals of Poland]. PWN, Warszawa, 1-364 [in Polish]. Sokolov V. E. 1973: Kožnyj pokrov mlekopitajušcich. Nauka, Moskwa, 1-428 [in Russian]. StatSoft, Inc. 2004: STATISTICA for Windows [Computer program manual]. Tulsa.
Accepted for print: 4.11.2006