INTRODUCTION
Shiga-like toxin-producing E. coli strains (STEC) for the first time were classify as humans pathogens for the first time in 1982 when their association with two independent outbreaks of hemorrhagic colitis (HC) in USA was established [15]. In the both outbreaks hamburgers were the source of infection. Since 1982 STEC infection have increasingly been reported all over the world. One of the reason for increasing frequency of STEC infection is improvement of diagnostic tools, as well as the epidemiological studies undertaken on a large scale [3]. On the other hand we can not exclude real increment of STEC infection as a result of spreading of these pathogens in the human and animal environment.
Shiga-like toxin-producing E. coli strains belong to many different O:H serotypes and the ability to shiga-like toxins synthesis have been described among other intestinal bacilli e.g. Enterobacter cloaceae, Citrobacter freundii, Aeromonas hydrophila [17].
HUMANS INFECTIONS
Not all STEC isolated from cattle, that are thought as the main reservoir of these bacteria, are pathogenic for humans. It seems that humans infection with STEC is limited to certain O:H serotypes, e.g. the first described O157:H7 among them. E. coli strains of O157:H7 serotype are usually producing two different shiga-like toxins: Stx1 and Stx2 apart from other virulence factors. Sporadic cases, and outbreaks of E. coli O157:H7 infection appears to be more common in USA, Canada, United Kingdom, and Japan [11]. In these countries serotype O157:H7 is responsible for 0.6 – 2.4% of all cases of diarrhea and 15 – 36% of cases HC among them, making the serotype the third of the most frequently isolated intestinal pathogen. Every year in USA STEC infection affects about 2000 persons with 250 fatal cases [15]. In Europe E. coli O157:H7 infection has been reported in United Kingdom, Ireland, Belgium, Germany, Czech Republic, but most frequently isolated STEC are non-O157 serotypes [5,11,12]. Shiga-like toxin-producing E. coli especially O111:H-, O26:H11, and O103:H2 serotypes and other are isolated from outbreaks as well as sporadic cases of diarrhea and hemolytic uremic syndrome (HUS). Diagnostic problems make the frequency of isolation of non-O157 STEC difficult to estimate. These strains have often been omitted in the routine diagnosis. Contrary to E. coli O157:H7 serotype, non-O157 STEC do not ferment sorbitol what makes MacConkey-sorbitol agar commonly used for detection of E. coli O157:H7 useless [3,11].
The number of STEC infection peaks in the summer and early autumn (above 60% cases). STEC infection occurs in all age groups, but the young are most often affected [3,17]. There are no differences in incidence with regard to sex. The case-fatality rate associated with HUS is even 30% among elderly patients and children less than 5 years old [3,11,17].
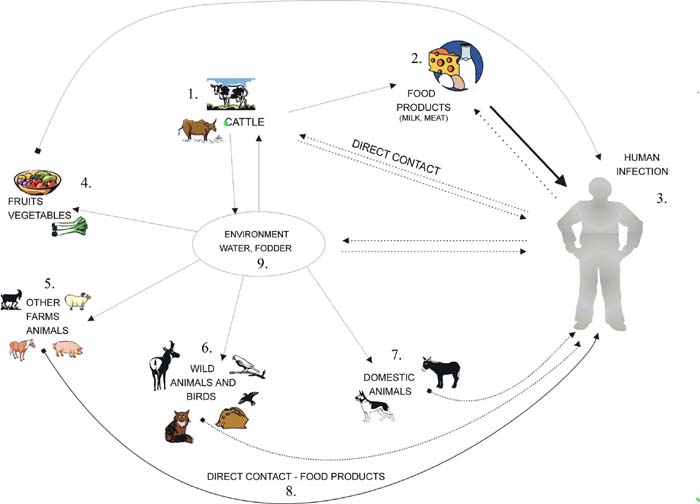
The main source of human infection is STEC contaminated food of animal origin: ground beef, pork, and goat’s, chickens, raw milk, and farmhouse cheeses, raw vegetables and fruits as well e.g. lettuce, radish sprouts, fresh-pressed apple cider, and squeezed fruits [5].
Sporadic cases of STEC infection have been linked to water: drinking of untreated water, swimming in pools contained with faces or lake water. The possibility person-to-person transmission of STEC and outbreaks occurring in nursing homes, schools, and day care centers indicate low infectious dose [2,11,15].
INFECTIOUS DOSE
The infectious dose for STEC is very low and depends on serotype. Fever than 10 bacterial cells of E. coli O157:H7 and O26:H11 serotypes are able to infect human. A low infectious dose of STEC correlates with acid resistance of STEC and their capacity to survive the low pH of gastric and colonize intestinal tract of humans. STEC are usually more acid tolerant than most other E. coli strains [2,8]. The infectious dose of STEC for animals has not been established but it was demonstrated that calves experimentally inoculated with 1010 E. coli O157:H7 cells became infected [2].
ANIMAL RESERVOIRS
Cattle are considered to be a reservoir of STEC. The numbers of E. coli O157:H7 in bovine faces range from 2.0 x 102 to 8.7x 104 bacterial cells per gram [1,4,10]. In the epidemiology of STEC in cattle environment different factors may play a role e.g. the age of cattle, diet, feed and water sources, animal density, season, wildlife exposure, geographical region, as well as management factors [13,17,19]. Colonization of cattle and other animals with STEC is generally asymptomatic although some STEC may produce enteric symptoms in young weaned calves [17]. It seems that only these STEC strains that possesses additional virulence properties apart from shiga-like toxins-production are pathogenic for cattle [11,17]. The lack of symptoms of infection among STEC-colonized cattle is probably due to the absence of specific for shiga-like toxins receptor – Gb3 (globotraiosyl ceramide) on intestinal epithelial cells [11]. Besides of that, some of STEC strains producing variant of shiga-like toxin 2 – Stx2e are responsible for pigs edema disease. These strains belong to limited number of E. coli serotypes: O138:K81, O139:K82, O141:K85 and they are not pathogenic for other animals and humans [6,17]. Cattle shed STEC intermittently and seasonally, and carriage within cattle appears to be transient and episodic. The magnitude of shedding of STEC among cattle increasing during summer and is similar to the patterns that are observed in humans infection. The shedding is greatest during the first two weeks postinoculation and decreases thereafter, but it can persist for as long as two years. Calves and heifers shed highest numbers of STEC for a longer duration [6,19]. The epidemiological data has showed domination and maintenance of one or several serotypes of STEC among herds, although persistence of some different serotypes is also possible. That could explain transmission of these subtypes or serotypes between cattle herds. Widespread contamination of water sources on farms seems to be one of the most important factor influencing the persistence of STEC among cattle. It has been shown that water contamination with c.a. 103 of E. coli O157:H7 cells per milliliter is sufficient to transmission and colonization of bulls herd [19].
| Figure 1. Epidemiology of STEC |
 |
The management practices can influence the level of STEC shedding in cattle [6,7,8]. It has been demonstrated that dietary stress increases shedding of STEC. In well-fed animals, the metabolic activities of rumen anaerobic bacterial flora produce a concentration of volatile fatty acids which suppress coliform number. During fasting protective influence of rumen is reduced and animals shed increased number of STEC [7]. That increases the risk of fecal contamination of meat at slaughter and vegetables that are fertilize with cattle manure. Cattle feeds can be fecal contaminated with STEC (e.g. by contamination from bird or rodent faces during storage or shipment) prior to delivery of feeds to the farm [9,14].
In the presence of proteinaceous materials STEC can tolerate dry environment conditions for several weeks. They can survive in bovine faces for longer than 42 days, and can replicate at temperatures between 25 to 30°C [8,14,20]. Apart from cattle asymptomatic carriage of STEC has been associated even though less frequently with other animals e.g. sheep, goats, dogs, horses, poultry, pigs, birds, deer’s and flies [5,7,17,19].
Widespread of STEC in animals environment makes carriage control and eradication difficult or even impossible, and isolation of infected with STEC animals seems to has no effect on limitation of transmission of these pathogens. Genes for shiga-like toxins are bacteriophage encoded and horizontal transfer of these phages among E. coli and other bacilli strains occurs in natural environments [16]. Furthermore intraintestinal transmission of the phages among native E. coli strains has been demonstrated in vivo on mouse model. The presence of high levels of phages carrying the stx2 gene as free particles has been shown in urban sewage. Taking into consideration that stx2-encoding phages can persist in sewage and are more resistant to chlorination, and pasteurization than bacteria, free phage particles may have a crucial role as a reservoir of shiga-like toxin gene stx2 in nature. Noteworthy is that Stx2 – producing E. coli strains are more frequently responsible for humans infection [16,17].
CONCLUSIONS
Increasing number of STEC humans infection is important problem for public health. Effective control strategies to limit STEC infection must consider the multiple points at which STEC or bacteriophages encoding shiga-like toxins genes can gain access to the human food chain. Since cattle are the main reservoir of STEC and food of animal origin is a source of humans infection, the strategies to reduce the prevalence of STEC in farm, slaughter arrangements, and hygiene practice are of special importance.
REFERENCES
Blanco M., Blanco J.E., Blanco J., Gonzales E.A., Mora A., Prado C., Fernandez L., Rio M., Ramos J., Alonso M.P., 1996. Prevalence and characteristic of Escherichia coli serotype O157:H7 and other verotoxin-producing E. coli in healthy cattle. Epidemiol. Infect., 117, 251–257. Brown C.A., Harmon B.G., Zhao T., Doyle M.P., 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol., 63, (1), 27–32. Chinyu S., Brandt L.J., 1995. Escherichia coli O157:H7 infections in humans. Ann. Intern. Med., 123, 698–714. Cobbold R., Desmarchelier P., 2002. Horizontal transmission of shiga toxin-producing Escherichia coli within groups of dairy calves. Appl. Environ. Microbiol., 68, (8), 4148–4152. Coia J.E., 1998. Clinical, microbiological and epidemiological aspects of Escherichia coli O157 infection. FEMS Immunol. Med. Microbiol., 20, 1–9. Cornick N.A., Booher S.L., Casey T.A., Moon H.W., 2000. Persistent colonization of sheep by Escherichia coli O157:H7 and other E. coli pathotypes. Appl. Environ. Microbiol., 66, (11), 4926–4934. Cray W.C., Casey T.A., Bosworth B.T., Rasmussen M.A., 1998. Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Appl. Environ. Microbiol., 64, (5), 1975–1979. Diez-Gonzalez F., Callaway T.R., Kizoulis M.G., Russell J.B., 1998. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science, 281, 1666–1668. Elder R.O., Keen J.E., Siragusa G.R., Barkocy-Gallagher G.A., Koohmaraie M., Laegreid W.W., 2000, Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. PNAS, 97, (7), 2999–3003. Galland J.C., Hyatt D.R., Crupper S.S., Acheson D.W., 2001. Prevalence, antibiotic susceptibility, and diversity of Escherichia coli O157:H7 isolates from a longitudinal study of beef cattle feedlots. Appl. Environ. Microbiol., 67, (4), 1619–1627. Griffin P., Tauxe R.V., 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev., 13, 60–98. Karch H., Bielaszewska M., Bitzan M., Schmidt H., 1999. Epidemiology and diagnosis of shiga toxin-producing Escherichia coli infections. Diagn. Microbiol. Infect. Dis., 43, 229–243. Lahti E., Ruoho O., Rantala L., Hänninen M.L., Honkanen-Buzalski T., 2003. Longitudinal study of Escherichia coli O157 in a cattle finishing unit. Appl. Environ. Microbiol., 69, (1), 554–561. Lynn T.V., Hancock D.D., Besser T.E., Harrison J.H., Rice D.H., Stewart N.T., Rowan L.L., 1998. The occurrence and replication of Escherichia coli in cattle feeds. J. Dairy Sci., 81, 1102–1108 Maidhof H., Guerra B., Abbas S., Elsheikha H.M., Whittam T.S., Beutin L., 2002. A multi resistant clone of shiga toxin-producing Escherichia coli O118:[H16] is spread in cattle and humans over different European countries. Appl. Environ. Microbiol., 68, (12), 5834–5842 Muniesa M., Jofre J., 1998. Abundance in sewage of bacteriophages that infect Escherichia coli O157:H7 and that carry the shiga toxin 2 gene. Appl. Environ. Microbiol., 64, (7), 2443–2448. Paton J.C., Paton A.W., 1998. Pathogenesis and diagnosis of shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev., 11, (3), 450–479. Renter D.G., Sargeant J.M., Oberst R.D., Samadpour M., 2003. Diversity, frequency, and persistence of Escherichia coli O157 strains from range cattle environments. Appl. Environ. Microbiol., 69, (1), 542–547 Shere J.A., Kaspar C.W., Bartlett K.J., Linden S.E., Norell B., Francey S., Shaefer D.M., 2002. Shedding of Escherichia coli O157:H7 in dairy cattle housed in a confined environment following waterborne inoculation. Appl. Environ. Microbiol., 68, (4), 1947–1954 Wang G., Zhao T., Doyle M.P., 1996. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl. Environ. Microbiol., 62, (7), 2567–2570.